تنگستن
 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| تنگستن | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| الاسم البديل | Wolfram, pronounced: /ˈwʊlfrəm/ (WUUL-frəm) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| التآصلات | α-tungsten (common), β-tungsten | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| المظهر | أبيض رمادي لامع | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الوزن الذري العياري Ar°(W) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| تنگستن في الجدول الدوري | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الرقم الذري (Z) | 74 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| المجموعة | 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الدورة | period 6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| المستوى الفرعي | d-block | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| التوزيع الإلكتروني | [Xe] 4f14 5d4 6s2[1] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الإلكترونات بالغلاف | 2, 8, 18, 32, 12, 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الخصائص الطبيعية | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الطور at د.ح.ض.ق | solid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نقطة الانصهار | 3695 K (3422 °س، 6192 °F) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نقطة الغليان | 6203 K (5930 °س، 10706 °ف) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الكثافة حين يكون سائلاً (عند ن.إ.) | 17.6 ج/سم³ | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| حرارة الانصهار | 52.31 kJ/mol[2][3] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| حرارة التبخر | 774 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| السعة الحرارية المولية | 24.27 J/(mol·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
ضغط البخار
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الخصائص الذرية | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الكهرسلبية | مقياس پاولنگ: 2.36 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| طاقات التأين |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نصف القطر الذري | empirical: 139 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نصف قطر التكافؤ | 162±7 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| خصائص أخرى | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| البنية البلورية | مكعب مركزي الجسم | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| سرعة الصوت قضيب رفيع | 173 W/(m·K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| المقاومة الكهربائية | 52.8 nΩ⋅m (at 20 °C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الترتيب المغناطيسي | المغناطيسية المسايرة[4] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| القابلية المغناطيسية | +59.0×10−6 cm3/mol (298 ك)[5] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| معامل يونگ | 411 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| معامل القص | 161 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| معاير الحجم | 310 GPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نسبة پواسون | 0.28 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| صلادة موز | 7.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| صلادة ڤيكرز | 3430–4600 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| صلادة برينل | 2000–4000 MPa | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| رقم كاس | 7440-33-7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| التاريخ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| التسمية | الاسم السويدي القديم لمعدن الشيليت، الذي عُزل منه؛ ويعني "الحجر الثقيل". | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الاكتشاف وأول عزل | خوان خوسيه إلهويار وفاوستو إلهويار[6] (1783) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| سمّاه | توربرن برگمان (1781) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| الرمز | "W": from Wolfram, originally from Middle High German wolf-rahm 'wolf's foam' describing the mineral wolframite[7] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| نظائر التنگستن | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
التنگستن (Tungsten)، (ويُسمى أيضاً ولفرام wolfram)[10][11] هو عنصر كيميائي يحمل الرمز W (من ألمانية: Wolfram) ورقمه الذري 74. وهو فلز يوجد طبيعياً على سطح الأرض بشكل شبه حصري في مركبات كيميائية مع عناصر أخرى. أُكتشف كعنصر مستقل عام 1781، وعُزل لأول مرة كفلز عام 1783. من أهم خاماته الشيليت والولفراميت، الذي يُعطي العنصر اسمه البديل.
يتميز هذا العنصر الحر بمتانته، وخاصةً أنه يمتلك أعلى درجة انصهار بين جميع العناصر المكتشفة باستثناء الكربون (الذي يتسامى عند الضغط العادي)، حيث ينصهر عند 3422°. كما أنه يمتلك أعلى درجة غليان، عند 5930°.[12] تبلغ كثافته 19.25 جراماً لكل سنتيمتر مكعب،[13] مقارنة باليورانيوم والذهب، وأعلى بكثير (حوالي 1.7 مرة) من الرصاص.[14] التنگستن عديد البلورات هو مادة هشة بطبيعتها[15][16] ومادة صلابة (في الظروف القياسية، عندما تكون غير مركبة)، مما يجعل تشكيله أمراً صعباً. ومع ذلك، فإن التنگستن النقي أحادي البلورة أكثر ليونة ويمكن قطعه بمنشار يدوي مصنوع من الصلب.[17]
يوجد التنگستن في العديد من السبائك، التي لها تطبيقات عديدة، بما في ذلك فتائل المصابيح المتوهجة، وأنابيب الأشعة السينية، وأقطاب اللحام بالقوس الكهربائي بالتنگستن، والسبائك الفائقة، والوقاية من الإشعاع. وتجعل صلابة التنگستن وكثافته العالية منه مناسباً للتطبيقات العسكرية في المقذوفات الخارقة. كما تُستخدم مركبات التنگستن غالباً كمحفزات صناعية.
التنگستن هو الفلز الوحيد في سلسلة الفلزات الانتقالية الثالثة المعروف بوجوده في الجزيئات الحيوية، حيث يوجد في أنواع قليلة من الجراثيم والعتائق. مع ذلك، يتداخل التنگستن مع استقلاب المولبدنم والنحاس، وهو سام إلى حد ما لمعظم أشكال الحياة الحيوانية.[18][19]
الخصائص
الخصائص الفيزيائية
التنگستن في حالته الخام هو فلز صلب رمادي فولاذي، غالباً ما يكون هشاً ويصعب تشكيله. أما التنگستن النقي أحادي البلورة فيحتفظ بصلابته (التي تفوق صلابة العديد من أنواع الصلب)، ويصبح قابلاً للطرق بدرجة كافية ليسهل تشكيله.[17] يُشكل التنگستن بالتطريق، أو السحب، أو البثق، لكن عملية التلبيد هي الأكثر شيوعاً. ويُستخدم التلبيد غالباً نظراً لارتفاع درجة انصهار التنگستن.
من بين جميع الفلزات في شكلها النقي، يتمتع التنگستن بأعلى نقطة الانصهار (3422°س)، وأقل ضغط بخار (عند درجات حرارة أعلى من 1650°س)، وأعلى مقاومة شد.[20] على الرغم من أن الكربون يبقى صلباً عند درجات حرارة أعلى من التنگستن، إلا أنه يتسامى عند الضغط الجوي بدلاً من الانصهار، لذا فهو لا يمتلك نقطة انصهار. علاوة على ذلك، فإن الطور البلوري، أكثر أطوار التنگستن استقراراً، لا يُظهر أي تحولات بنيوية ناتجة عن الضغط العالي حتى ضغوط تصل إلى 364 گيگا پاسكال على الأقل.[21] يتميز التنگستن بأقل معامل تمدد حراري بين جميع الفلزات النقية. ويعود انخفاض معامل التمدد الحراري وارتفاع درجة انصهاره ومقاومته للشد إلى قوة الروابط التساهمية المتشكلة بين ذرات التنگستن بواسطة إلكترونات 5d.[22] يؤدي خلط كميات صغيرة من التنگستن مع الصلب إلى زيادة كبيرة في صلابته.[14]
يوجد التنگستن في شكلين بلوريين رئيسيين: ألفا وبيتا. يتميز الشكل ألفا ببنية مكعبة مركزية الجسم، وهو الشكل الأكثر استقراراً. تُسمى بنية طور بيتا بالتنگستن المكعب A15؛ وهو شبه مستقر، لكنه قد يتعايش مع طور ألفا في الظروف المحيطة نتيجةً للتخليق غير المتوازن أو التثبيت بواسطة الشوائب. على عكس طور ألفا الذي يتبلور في حبيبات متساوية الأبعاد، يُظهر طور بيتا هيئة عمودية. يتميز طور ألفا بثلث المقاومة الكهربائية[23] ودرجة حرارة انتقال الموصلية الفائقة TC أقل بكثير مقارنةً بالطور بيتا: حوالي 0.015 كلڤن مقابل 1-4 كلڤن؛ يسمح خلط الطورين بالحصول على قيم TC متوسطة.[24][25] يمكن أيضًا رفع قيمة TC عن طريق خلط التنجستن مع فلز آخر (على سبيل المثال 7.9 كلگن لسبيكة التنگستن-تكنتيوم).[26] تُستخدم سبائك التنگستن هذه أحياناً في الدوائر فائقة التوصيل ذات درجة الحرارة المنخفضة.[27][28][29]
النظائر
يتكون التنگستن التواجد بشكل طبيعي من أربعة نظائر مستقرة (182W، 183W، 184W، و186W) ونظير مشع واحد طويل العمر للغاية، 180W. نظرياً، يمكن أن تتحلل جميع النظائر الخمسة إلى نظائر العنصر 72 (الهافنيوم) عن طريق انحلال ألفا، لكن لوحظ أن 180W فقط هو الذي يفعل ذلك، بنصف عمر يبلغ (1.8±0.2)×1018 سنة؛[30][31] في المتوسط، ينتج عن هذا حوالي اثنين من اضمحلالات ألفا بمقدار 180 واط لكل جرام من التنگستن الطبيعي سنوياً.[32] يُعادل هذا المعدل نشاطاً نوعياً يبلغ حوالي 63 ميكروبكرل لكل كيلوجرام. هذا المعدل أقل بكثير من معدل الملحوظ المُلاحظ في الكربون أو الپوتاسيوم الموجودين على الأرض، واللذين يحتويان أيضاً على كميات ضئيلة من النظائر المشعة طويلة العمر. لطالما اعتُقد أن البيزموث غير مشع، لكن نظيره، البيزموث-209، (أطول نظائره عمراً) ينحل في الواقع بنصف عمر يبلغ 2.01 × 10^19 سنة، أي أبطأ بعشر مرات تقريباً من نظير التنگستن-180. مع ذلك، ولأن البيزموث الموجود في الطبيعة يتكون بنسبة 100% من البيزموت-209، فإن نشاطه النوعي أعلى من نشاط التنگستن الطبيعي، والذي يبلغ 3 ملي بكرل لكل كيلوجرام. لم يُلاحظ انحلال النظائر الأخرى الموجودة بشكل طبيعي للتنگستن، مما يحد من عمر النصف لها ليكون على الأقل 4×1021 سنة.
تم تحديد خصائص 34 نظيراً مشعاً اصطناعياً آخر للتنگستن، وأكثرها استقراراً هو 181W بنصف عمر يبلغ 121.2 يوماً، و185W بنصف عمر يبلغ 75.1 يوماً، و188W بنصف عمر يبلغ 69.4 يوماً، و178W بنصف عمر يبلغ 21.6 يوماً، و187W بنصف عمر يبلغ 23.72 ساعة.[32] جميع النظائر المنحلة اشعاعياً المتبقية لها عمر نصف أقل من 3 ساعات، ومعظمها لها عمر نصف أقل من 8 دقائق.[32] يمتلك التنگستن أيضاً 12 مصاوغ نووي، وأكثرها استقراراً هو 179mW (t1/2 6.4 دقيقة).
الخصائص الكيميائية
التنگستن عنصر غير نشط في الغالب: فهو لا يتفاعل مع الماء، ومقاوم لمعظم الأحماض والقواعد، ولا يتفاعل مع الأكسجين أو الهواء في درجة حرارة الغرفة. عند درجات حرارة مرتفعة (أي عندما يكون متوهجاً)، يتفاعل مع الأكسجين لتكوين المركب ثلاثي الأكسيد، أكسيد التنگستن(VI)، WO3. ومع ذلك، فإنه يتفاعل مباشرة مع الفلور (F2) في درجة حرارة الغرفة لتكوين فلوريد التنگستن السداسي (WF6)، وهو غاز عديم اللون. عند حوالي 250 درجة مئوية، يتفاعل مع الكلور أو البروم، وفي ظروف حارة معينة يتفاعل مع اليود. مسحوق التنگستن الناعم قابل للاشتعال التلقائي.[33][34]
حالة الأكسدة الرسمية الأكثر شيوعاً للتنگستن هي +6، لكنه يُظهر جميع حالات الأكسدة من -2 إلى +6.[34][35] يتحد التنگستن عادة مع الأكسجين لتكوين أكسيد التنگستيك الأصفر، WO3، والذي يذوب في المحاليل القلوية المائية لتكوين أيونات التنگستات، WO2−4.
تنتج كربيدات التنگستن (W2C and WC) بتسخين مسحوق التنگستن مع الكربون. يتميز W2C بمقاومته للهجوم الكيميائي، على الرغم من تفاعله الشديد مع الكلور لتكوين كلوريد التنگستن السداسي (WCl6).[14]
في المحلول المائي، يُعطي التنگستات أحماضاً غير متجانسة وأنيونات فلزية عديدة الأكسجين في الظروف المتعادلة والحمضية. عند معالجة التنگستات تدريجياً بالحمض، فإنه يُنتج أولاً أنيون "فوق التنگستات إيه" القابل للذوبان وشبه المستقر، W 7O6−24، والذي يتحول بمرور الوقت إلى أنيون "فوق التنگستات بي" الأقل قابلية للذوبان، H 2W 12O10−42.[36] يؤدي المزيد من المعالجة بالحمض إلى إنتاج أنيون ما بعد التنگستات شديد الذوبان، H 2W 12O6−40، بعد ذلك، يتحقق التوازن. يوجد أيون ما بعد التنگستات على شكل عنقود متناظر من اثنتي عشرة وحدة من التنگستن والأكسجين، يُعرف بأنيون كگين. توجد العديد من الأنيونات الفلزية عديدة الأكسجين الأخرى كأنواع شبه مستقرة. يؤدي إدخال ذرة مختلفة، مثل الفوسفور، بدلًا من ذرتي الهيدروجين المركزيتين في ما بعد التنگستات إلى إنتاج مجموعة واسعة من الأحماض غير المتجانسة، مثل حمض الفوسفوتنگستيك H3PW12O40.
يمكن أن يشكل أكسيد التنگستن الثلاثي مركبات الإقحام مع الفلزات القلوية. تُعرف هذه المركبات بالبرونزات؛ ومن الأمثلة على ذلك برونز الصوديوم التنگستن.
في الحالة الغازية، يشكل التنگستن جزيئاً ثنائي الذرة W2. تتميز هذه الجزيئات برابطة سداسية بين ذرات التنگستن - وهي أعلى رتبة رابطة معروفة بين الذرات المستقرة.[37][38]
التاريخ
عام 1781، اكتشف كارل ڤيلهلم شيله أنه يمكن صنع حمض جديد، وهو حمض التنگستيك، من الشيليت (الذي كان يسمى في ذلك الوقت التنگستن).[39][40] اقترح شيله و توربرن برگمان أنه ربما من الممكن الحصول على معدن جديد عن طريق اختزال هذا الحمض.[41] في عام 1783، اكتشف خوسيه وفاستو إلهاير حمضاً مصنوعاً من الولفراميت مطابقاً لحمض التنگستيك. وفي وقت لاحق من ذلك العام، في الجمعية الملكية الباسكية، نجح الأخوان في عزل التنگستن عن طريق اختزال هذا الحمض بالفحم النباتي، ويُنسب إليهما اكتشاف هذا العنصر (وقد أطلقوا عليه اسم "ولفرام" أو "ڤولفرام").[42][43][44][45][46]
برزت القيمة الاستراتيجية للتنگستن في أوائل القرن العشرين. وفي عام 1912، تحركت السلطات البريطانية لتحرير منجم كاروك من شركة كمبريان للتعدين المملوكة لألمانيا، وأثناء الحرب العالمية الأولى، قيدت وصول الألمان إلى مناطق أخرى.[47] خلال الحرب العالمية الثانية، لعب التنگستن دوراً أكثر أهمية في التعاملات السياسية الخلفية. وتعرضت الپرتغال، بصفتها المصدر الأوروپي الرئيسي لهذا العنصر، لضغوط من كلا الجانبين بسبب رواسب خام الولفراميت في پاناسكويرا. وقد جعلت خصائص التنگستن المرغوبة، مثل مقاومته لدرجات الحرارة العالية وصلابته وكثافته وقدرته على تقوية السبائك، منه مادة خام هامة لصناعة الأسلحة،[48][49] سواء كمكون من مكونات الأسلحة والمعدات أو يستخدم في الإنتاج نفسه، على سبيل المثال، في أدوات القطع المصنوعة من كربيد التنگستن لتصنيع الصلب. يُستخدم التنگستن الآن في العديد من التطبيقات الأخرى مثل أوزان التوازن في الطائرات ورياضة السيارات، والسهام، وأدوات مقاومة الاهتزاز، والمعدات الرياضية.
يُعدّ التنگستن عنصراً فريداً من نوعه بين العناصر الأخرى، إذ كان موضوعاً لإجراءات براءات الاختراع. ففي عام 1928، رفضت محكمة أمريكية محاولة شركة جنرال إلكتريك لتسجيل براءة اختراع له، وألغت براءة الاختراع الأمريكية رقم 1082933 الممنوحة عام 1913 لوليام كولدج.[50][51][52]
ويُقترح أنه قد عُثر على بقايا من الولفرام فيما قد يكون حديقة عالم الفلك والخيميائي تيكو براهى.[53]
التسمية
يُستخدم اسم تنگستن (tungsten، والذي يعني 'الحجر الثقيل' في اللغة السويدية، وكان الاسم السويدي القديم لمعدن الشيليت ومعادن أخرى ذات كثافة مماثلة) في الإنگليزية والفرنسية والعديد من اللغات الأخرى كاسم للعنصر، لكن يُستخدم اسم الولفرام (أو الڤولفرام) في معظم اللغات الأوروپية (وخاصة الجرمانية والسلاڤية) وهو مشتق من معدن الولفراميت ، وهو أصل الرمز الكيميائي W.[17] اسم الولفراميت مشتق من الكلمة الألمانية wolf rahm (أي "سخام الذئب"، أو "كريمة الذئب")، وهو الاسم الذي أطلقه يوهان گوتشالك ڤالريوس على التنگستن عام 1747. وهذا الاسم بدوره مشتق من الكلمة اللاتينية lupi spuma، وهو الاسم الذي استخدمه گيورگ أگريكولا للمعدن عام 1546، والذي يُترجم إلى العربية 'زبد الذئب'، في إشارة إلى الكميات الكبيرة من القصدير التي يستهلكها المعدن أثناء استخراجه، كما لو كان المعدن يلتهمه كالذئب.[7] تُتبع هذه التسمية تقليداً للأسماء الملونة التي كان عمال المناجم في جبال الخام يطلقونها على المعادن المختلفة، انطلاقاً من خرافة مفادها أن بعض المعادن التي بدت وكأنها تحتوي على معادن ثمينة معروفة آنذاك، لكن عند استخراجها كانت "ملعونة" بطريقة ما. يستمد كل من الكوبالت (من كوبولد)، والپيتشبلند (من كلمة بلندن الألمانية التي تعني اللمعان أو الخداع)، والنيكل (من "أولد نيك") أسماءها من نفس التعبير الدارج بين عمال المناجم.
التواجد
لم يُعثر على التنگستن في الطبيعة حتى الآن في صورته النقية.[54] بدلاً من ذلك، يوجد التنگستن بشكل رئيسي في معدني الولفراميت والشيليت.[54] الولفراميت هو تنگستات الحديد-المنگنيز (Fe,Mn)WO
4، وهو محلول صلب من معدني الفربريت (FeWO4)، والهوبنريت (MnWO4)، بينما الشيليت هو تنگستات الكالسيوم (CaWO4). أما معادن التنگستن الأخرى فتتراوح وفرتها بين المتوسطة والنادرة جداً، وهي عديمة القيمة الاقتصادية تقريباً.
الإستخدامات
وفتائل أسلاكه تستخدم في الغالبية العظمى من المصابيح الكهربائية المتوهجة العادية ويشيع استخدامها كذلك في إضاءة المصانع كإلكترودات في لمبات القوس الكهربى، ويستخدم أيضا في معدات لحام القوس بالتنگستن و الغاز الخامل (TIG or GTAW) كإلكترود معمّر يقاوم الانصهار. أما الاستخدام الأكثر شيوعا للتنگستن فهو مركب كربيد التنگستن الداخل في صناعة لقم الثقب والتشغيل وعدد القطع. ويجد كذلك استخدامات كمزلق و مقاوم الأكسدة في البطانات والفوهات لمقاومة البرى وكطلاء واقى وغير ذلك من طرق الاستخدام، ويوجد التنگستن كذلك في أحبار الطباعة و شاشات الأشعة السينية وكيماويات التصوير وفى تجهيز المنتجات البترولية واختبار مقاومة اللهب للمنسوجات. ويستخدم أيضا لمقاومتة وكثافته العاليتين في تطبيقات تتراوح بين ثقالات في دوارات الطائرات المروحية وكقذائف الأسلحة وبين رؤوس مضارب الجولف.
النظائر
 مقالة مفصلة: نظائر التنگستن
مقالة مفصلة: نظائر التنگستن
يتكون التنگستن الموجود بشكل طبيعي من أربعة نظائر مستقرة (182W، 183W، 184W، و 186W) ونظير مشع واحد طويل العمر للغاية، 180W. نظريًا، يمكن أن تتحلل جميع العناصر الخمسة إلى نظائر للعنصر 72 (الهافنيوم) عن طريق انحلال ألفا، لكن لوحظ فقط أن 180W يفعل ذلك، بنصف عمر يبلغ (1.8±0.2)×1018 سنة؛[55][56] في المتوسط، ينتج عن هذا حوالي اثنين من انحلال ألفا بمقدار 180 واط لكل جرام من التنگستن الطبيعي سنوياً.[32] يُعادل هذا المعدل نشاطاً نوعياً يبلغ حوالي 63 ميكروبكريل لكل كيلوجرام. هذا المعدل أقل بكثير من معدل التحلل المُلاحظ في الكربون أو الپوتاسيوم الموجودين على الأرض، واللذين يحتويان أيضاً على كميات ضئيلة من النظائر المشعة طويلة العمر. لطالما اعتُقد أن البيزموث غير مشع، لكن نظير البيزموث-209 (أطول نظائره عمراً) يتحلل في الواقع بنصف عمر يبلغ 2.01×1019 سنة، أي أبطأ بعشر مرات تقريباً من نظير التنگستن-180. مع ذلك، ولأن البيزموث الموجود في الطبيعة يتكون بنسبة 100% من البيزموث-209، فإن نشاطه النوعي أعلى من نشاط التنگستن الطبيعي، والذي يبلغ 3 ملي بكريل لكل كيلوجرام. لم يُلاحظ تحلل النظائر الأخرى الموجودة بشكل طبيعي للتنگستن، مما يحد من عمر النصف لها ليكون على الأقل 4×1021 سنة.
تم تحديد خصائص 34 نظيراً مشعاً اصطناعياً آخر للتنگستن، وأكثرها استقراراً هو 181W بنصف عمر يبلغ 121.2 يوماً، و185W بنصف عمر يبلغ 75.1 يوماً، و188W بنصف عمر يبلغ 69.4 يوماً، و178W بنصف عمر يبلغ 21.6 يوماً، و187W بنصف عمر يبلغ 23.72 ساعة.[32] جميع النظائر المشعة المتبقية لها أنصاف أعمار أقل من 3 ساعات، ومعظمها له أنصاف أعمار أقل 8 دقائق.[32] للتنگستن أيضاً 12 مصاوغ نووي، وأكثرها استقراراً هو 179mW (t1/2 6.4 دقيقة).
مركباته
للتنگستن ثلاثة أكاسيد هي ثنائي أكسيد التنگستن WO2 ويحضر بإرجاع ثالث أكسيد التنگستن بالهيدروجين، وأكسيد التنگستن الأزرق W2O3 وثلاثي أكسيد التنگستن WO3 وهو أهم أكاسيده، وهو مسحوق أصفر لا ينحل في الماء وينحل في القلويات مكوّناً التنگستات.[57]
ومن مركبات التنگستن أيضاً حمض التنگستيك WO4H2 وهو مسحوق أصفر أو أخضر مصفر، غير ذواب في الماء، وينحل ببطء في محاليل القلويات. ومن مركباته أيضاً تنگستات الصوديوم Na2WO4, 2H2O وهو مسحوق أبيض ينحل في الماء ولا ينحل في الغول ويستعمل لتحضير النسج التي لا تحترق والتي لا ينفذ منها الماء.
تحضيره
إن المصدرين الرئيسيين اللذين يعوَّل عليهما في تحضير التنغستن هما الشيليت والولفراميت، ويحضر التنگستن منهما بطرائق متعددة منها أن يسخن الفلز مع كربونات الصوديوم ثم تستخلص تنگستات الصوديوم الناتجة بالماء وترسب بعد ذلك بإضافة كلور الكلسيوم بحالة تنغستات الكلسيوم وهذه الأخيرة تعالج بحمض كلور الماء فينفصل أكسيد التنگستن WO3 الذي يرجع بالهدروجين بدرجة حرارة 1200ْس.
الانتاج والتواجد
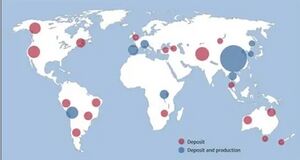
يتواجد التنگستن في معادن الولفراميت (تنگستات الحديد-المنگنيز، FeWO4/MnWO4)، الشيليت (تنگستات الكالسيوم، CaWO4)، الفربريت والهوبنريت. وتوجد الرواسب الرئيسية لهذه المعادن في الصين (التي تملك 57% من احتياطيات العالم)، روسيا، النمسا والپرتغال، حسب المسح الجيولوجي البريطاني. يُنتج الفلز تجارياً باختزال اكسيد التنگستن باستخدام الهيدروجين أو الكربون.
الاحتياطيات
تبلغ احتياطيات العالم من التنگستن 3.200.000 طن؛ وتقع معظمها في الصين (1.800.000 طن)، كندا (290.000 طن)،[59] روسيا (160.000 طن)، ڤيتنام (95.000 طن) وبوليڤيا. حتى عام 2017، كانت الصين وڤيتنام وروسيا الموردين الرئيسيين للتنگستن، حيث بلغت صادراتهم 79.000 و7.200 و3.100 طن على التوالي. وكانت كندا قد أوقفت الإنتاج في أواخر عام 2015 بسبب إغلاق منجم التنگستن الوحيد لديها. في المقابل، زادت ڤيتنام إنتاجها بشكل ملحوظ خلال عقد 2010، بفضل التحسين الكبير الذي أجرته على عمليات التكرير المحلية، متجاوزة بذلك روسيا وبوليڤيا.[60]
لا تزال الصين رائدة العالم ليس فقط في إنتاج منتجات التنگستن، بل أيضاً في تصديرها واستهلاكها. ويتزايد إنتاج التنگستن تدريجياً خارج الصين نتيجةً لارتفاع الطلب. في الوقت نفسه، تخضع إمداداته من الصين لرقابة صارمة من قبل الحكومة الصينية، التي تكافح التعدين غير القانوني والتلوث المفرط الناجم عن عمليات التعدين والتكرير.[61]
يوجد منجم كبير لخام التنگستن على حافة دارتمور في المملكة المتحدة، وقد استُغل خلال الحربين العالميتين الأولى والثانية تحت اسم منجم همردون. وبعد ارتفاع أسعار التنگستن، أُعيد تشغيل هذا المنجم عام 2014،[62] لكن أنشطته توقفت عام 2018.[63]
داخل الاتحاد الأوروپي، يعد منجم فلبرتال شيليت في النمسا أحد المناجم القليلة المنتجة للتنگستن.[64] تُعد الپرتغال واحدة من أهم منتجي التنگستن في أوروپا، حيث بلغ إنتاجها من التنگستن في المركزات المعدنية 121.000 طن في الفترة من 1910 حتى 2020، وهو ما يمثل حوالي 3.3% من الإنتاج العالمي.[65]
يعتبر التنگستن من المعادن المتنازع عليها بسبب ممارسات التعدين غير الأخلاقية التي لوحظت في جمهورية الكونغو الديمقراطية.[66][67]
منجم سانگدونگ في كوريا الجنوبية، وهو أحد أكبر مناجم التنگستن في العالم حيث يُقال إن 7.890.000 طن من التنگستن عالي الجودة مدفونة فيه، أُغلق عام 1994 بسبب انخفاض الربحية، لكن أُعيد تسجيل حقوق التعدين للمنجم ومن المقرر أن يستأنف أنشطته عام 2024.[68][69]
إحكام سيطرة الصين على الإنتاج
في 4 فبراير 2025، طبقت بكين نظام ترخيص تصدير التنگستن. والذي لا يُعتبر حظراً، بل مجرد إجراءات ورقية. نتيجة لذلك لم يحصل المستوردون الأمريكيون على أي تراخيص تصدير طوال العام. لم تتم الموافقة على كيلوجرام واحد، الذي يعد بمثابة عقوبات بيروقراطية هادئة لكنها مدمرة. تسيطر الصين على 82.7% من الإنتاج العالمي للتنگستن، أما الولايات المتحدة فلا تُنتج التنگستن على الإطلاق.
يُستخدم التنگستن في، صناعة الذخيرة الخارقة للدروع، وصلات أشباه الموصلات، وجميع أدوات القطع المستخدمة في التصنيع. عدم وجود التنگستن يعني عدم وجود طلقات الرصاص ولا الرقائق وتعطل عمليات التصنيع. هذا وقد كشف الپنتاگون أن مخزون الجيش الأمريكي من الأسلحة الخارقة للدروع لا يكفي لتلبية متطلبات القتال إلا لمدة 90 يوماً. وتواجه مصانع أشباه الموصلات انخفاضاً في بدء إنتاج الرقائق خلال 6-8 أسابيع من النقص الحاصل. وقد ارتفعت الأسعار بنسبة 200% هذا العام وحده. ويحظر قانون تفويض الدفاع الوطني لعام 2027 استخدام التنگستن الصيني في عقود الدفاع.
لن تصل مناجم الغرب إلى مرحلة الإنتاج قبل عامي 2028-2030، ومن ثم لا يمكن سد الفجوة الناجمة عن استخدام التنگستن الصيني في الوقت المناسب. ويأتي التقييد الصيني لتصدير التنگستن كتكملة للخطة التي بدأتها بتقييد تصدير الگاليوم والجرمانيوم والأنتيمون.[70]
الاستخلاص
يُستخلص التنگستن من خاماته على عدة مراحل. يُحوّل الخام في النهاية إلى ثلاثي أكسيد التنگستن (WO3)، والذي يُسخّن مع الهيدروجين أو الكربون لإنتاج مسحوق التنگستن.[41]
نظراً لارتفاع درجة انصهار التنگستن، فإن صبات سبائك التنگستن غير مجدية تجارياً. بدلاً من ذلك، يُخلط مسحوق التنگستن مع كميات صغيرة من مسحوق النيكل أو فلزات أخرى، ثم يُلبّد. خلال عملية التلبيد، ينتشر النيكل داخل التنگستن، مُنتجاً سبيكة.
يمكن أيضًا استخلاص التنگستن طريق اختزال الهيدروجين لسداسي فلوريد التنگستن (WF6):
- WF6 + 3 H2 → W + 6 HF
أو عن طريق التحلل الحراري:[71]
- WF6 → W + 3 F2 (ΔHr = +)
لا يُتداول التنگستن كأحد سلع العقود الآجلة، ولا يمكن تتبعه في البورصات مثل بورصة لندن للمعادن. غالباً ما تستخدم صناعة التنگستن مصادر تسعير مستقلة، مثل أرگوس ميديا أو متال بولتين، كأساس للعقود.[72] عادةً ما تُذكر الأسعار لمركزات التنگستن أوWO3.[60]
التطبيقات


يُستهلك ما يقارب نصف التنگستن في إنتاج المواد الصلبة، وتحديداً كربيد التنگستن، بينما يُستخدم الجزء الأكبر المتبقي في صناعة السبائك والصلب. ويُستخدم أقل من 10% منه في مركبات كيميائية أخرى.[73] نظراً لارتفاع درجة حرارة التحول من الليونة إلى الهشاشة في التنگستن، تُصنّع منتجاته تقليدياً باستخدام تقنيات متالورجيا المساحيق، والتلبيد بالپلازما الشرارية، والترسيب الكيميائي للبخار ، والكبس الساخن متساوي الضغوط، واللدائن الحرارية. ويُعدّ الصهر الانتقائي بالليزر بديلاً تصنيعياً أكثر مرونة، وهو شكل من أشكال الطباعة المجسمة، ويتيح إنشاء أشكال ثلاثية الأبعاد معقدة.[74]
التطبيقات الصناعية
يُستخدم التنگستن بشكل أساسي في إنتاج المواد الصلبة القائمة على كربيد التنگستن (WC)، وهو أحد أقسى أنواع الكربيدات. يُعد كربيد التنگستن موصلاً كهربائياً فعالاً، بينما يُعدّ ثنائي كربيد التنگستن W2C أقل كفاءة في التوصيل الكهربائي. يُستخدم كربيد التنگستن في صناعة المواد الكاشطة المقاومة للتآكل، وأدوات القطع الكربيدية مثل السكاكين، والمثاقب، والمناشير الدائرية، وإسطمبات التشكيل، وأدوات آلات التفريز والمخارط المستخدمة في تشغيل المعادن، والأخشاب، والتعدين، والبترول، والبناء.[14] الأدوات المصنوعة من الكربيد هي في الواقع مركب من السيراميك والفلز، حيث يعمل الكوبالت المعدني كمادة (مصفوفة) رابطة لتثبيت جزيئات كربيد التنگستن في مكانها. يمثل هذا النوع من الاستخدام الصناعي حوالي 60% من استهلاك التنگستن الحالي.[75]
تقوم صناعة المجوهرات بصنع الخواتم من كربيد التنگستن المتلبد، ومركبات كربيد التنگستن/الفلز، وكذلك التنگستن الفلزي.[76] تستخدم خواتم كربيد التنگستن/الفلز المركب النيكل كمادة أساسية معدنية بدلاً من الكوبالت، لأنه يكتسب بريقاً أعلى عند التلميع. أحياناً يشير المصنّعون أو تجار التجزئة إلى كربيد التنگستن كفلز، لكنه في الواقع سيراميك.[77] بسبب صلابة كربيد التنگستن، تتميز الخواتم المصنوعة من هذه المادة بمقاومة عالية للتآكل، وتحافظ على بريقها لفترة أطول من الخواتم المصنوعة من التنگستن الفلزي. مع ذلك، فإن خواتم كربيد التنگستن هشة، وقد تتشقق تحت الضربات القوية.[78]
السبائك
تساهم صلابة التنگستن ومقاومته للحرارة في صناعة سبائك مفيدة. ومن الأمثلة الجيدة على ذلك صلب القطع السريع، الذي قد يحتوي على ما يصل إلى 18% من التنگستن.[79] إن درجة انصهار التنگستن العالية تجعله مادة جيدة لتطبيقات مثل فوهات الصواريخ، على سبيل المثال في الصاروخ الباليستي يوجيإم-27 پولاريس الذي يُطلق من الغواصات.[80]
تُستخدم سبائك التنگستن في مجموعة واسعة من التطبيقات، بما في ذلك صناعات الطيران والفضاء والسيارات والتدريع الإشعاعي.[81] تُستخدم السبائك الفائقة التي تحتوي على التنگستن، مثل الهاستلوي والستلايت، في شفرات التوربينات والأجزاء والطلاءات المقاومة للتآكل.
تُعدّ مقاومة التنگستن للحرارة ميزة تجعله مفيداً في تطبيقات اللحام القوسي عند دمجه مع معدن آخر عالي التوصيل الحراري مثل الفضة أو النحاس. توفر الفضة أو النحاس التوصيلية اللازمة، بينما يسمح التنگستن لقضيب اللحام بتحمل درجات الحرارة العالية في بيئة اللحام القوسي.[82]
المغناطيسات الدائمة
استُخدم صلب التنگستن المُقسّى (المارتنسيتي) (بنسبة تنگستن تتراوح بين 5.5% و7.0% تقريبًا، ونسبة كربون تتراوح بين 0.5% و0.7%) في صناعة المغناطيسات الدائمة الصلبة، نظراً لمغناطيسيته المتبقية العالية ومقاومته، كما أشار جون هوپكنسون (1849-1898) في وقت مبكر من عام 1886. وتتأثر الخصائص المغناطيسية للفلزات أو السبائك بشدة بالبنية المجهرية. فعلى سبيل المثال، على الرغم من أن عنصر التنگستن ليس مغناطيسياً حديدياً (بينما الحديد كذلك)، إلا أنه عند وجوده في الصلب بهذه النسب، فإنه يُثبّت طور المارتنسيت، الذي يتميز بمغناطيسية حديدية أكبر من طور (أو الفريت (الحديد) نظراً لمقاومته الأكبر لحركة جدران النطاقات المغناطيسية.
التطبيقات العسكرية
يُستخدم التنگستن، الذي يُسبك عادةً مع النيكل أو الحديد أو الكوبالت لتكوين سبائك ثقيلة، في المقذوفات المخترقة بالطاقة الحركية كبديل لليورانيوم المنضب، في التطبيقات التي يُشكل فيها النشاط الإشعاعي لليورانيوم مشكلة حتى في حالته المنضبة، أو عندما لا تكون خصائص اليورانيوم الإضافية ذاتية الاشتعال مرغوبة (على سبيل المثال، في رصاصات الأسلحة الصغيرة العادية المصممة لاختراق الدروع الواقية). وبالمثل، استُخدمت سبائك التنگستن أيضاً في القذائف، القنابل اليدوية، والصواريخ، لإنتاج شظايا تفوق سرعة الصوت. استخدمت ألمانيا التنگستن أثناء الحرب العالمية الثانية لإنتاج قذائف لمدافع مضادة للدبابات، معتمدةً على ماسورة تضييق گرليتش لتحقيق سرعة فوهة عالية جداً واختراق معزز للدروع، وذلك باستخدام مدفعية ميدانية صغيرة العيار وخفيفة الوزن نسبياً. كانت هذه الأسلحة فعالة للغاية، إلا أن نقص التنگستن المستخدم في لب القذيفة، والذي نتج جزئياً عن أزمة الولفرام، حدّ من استخدامها.[citation needed]
كما أُستخدم التنگستن في المتفجرات الفلزية الخاملة الكثيفة، والتي تستخدمه كمسحوق كثيف لتقليل الأضرار الجانبية مع زيادة فتك المتفجرات ضمن نطاق صغير.[83]
التطبيقات الكيميائية
رباعي كبريتيد التنگستن هو مشحم عالي الحرارة، ويتكون من مكونات المحفزات لعملية نزع الكبريت المهدرج.[84] يُستخدم MoS2 بشكل أكثر شيوعاً لمثل هذه التطبيقات.[85]
تُستخدم أكاسيد التنگستن في طلاءات السيراميك، بينما تُستخدم تنگستات الكالسيوم/المغنسيوم على نطاق واسع في الإضاءة الفلورية. وتُستخدم بلورات التنگستات ككواشف وميضية في الفيزياء النووية والطب النووي. كما تُستخدم أملاح أخرى تحتوي على التنگستن في الصناعات الكيميائية والدباغة.[20] يُدمج أكسيد التنگستن (WO3) في محفزات الاختزال التحفيزي الانتقائي (SCR) المستخدمة في محطات توليد الطاقة التي تعمل بالفحم. تُحوّل هذه المحفزات أكاسيد النيتروجين (NOx) إلى نيتروجين (N2) وماء (H2O) باستخدام الأمونيا (NH3). يُساهم أكسيد التنگستن في تعزيز القاومة الفيزيائية للمحفز وإطالة عمره.[86] تُعد المحفزات المحتوية على التنگستن واعدة في العمليات الإيپوكسيدية،[87] oxidation,[88] وتفاعلات التحلل الهيدروجيني.[89] تُعد أحماض التنگستن غير المتجانسة مكوناً رئيسياً للمحفزات متعددة الوظائف.[90] يمكن استخدام التنگستات كمحفزات ضوئية،[91] بينما يُستخدم كبريتيد التنگستن كمحفز كهربائي.[92]
استخدامات متخصصة
تشمل التطبيقات التي تتطلب كثافته العالية الأوزان، الأوزان الموازنة، وعوارض التوازن لليخوت، ووزن التوازن الخلفي للطائرات التجارية، وأوزان الدوار للمروحيات المدنية والعسكرية، وكوزن توازن في سيارات السباق لناسكار وفورميولا 1.[93] نظراً لكثافته التي تقل قليلاً عن ضعف كثافة الرصاص، يُعتبر التنگستن بديلاً (وإن كان أغلى ثمناً) لأثقال الصيد الرصاصية. كما يُستخدم اليورانيوم المنضب لهذه الأغراض، نظراً لكثافته العالية المماثلة. استُخدمت كتل من التنگستن تزن 75 كيلوجرام "كأجهزة موازنة" في مركبة دخول الغلاف الجوي لمركبة مختبر علوم المريخ 2012. وهو مادة مثالية للاستخدام كأداة تثبيت للمسامير، حيث يمكن الحصول على الكتلة اللازمة لنتائج جيدة في قضيب مضغوط. تُستخدم سبائك التنگستن عالية الكثافة مع النيكل أو النحاس أو الحديد في صناعة لعب سهام الرشق عالية الجودة[94] (للسماح بقطر أصغر وبالتالي تجميعات أكثر تقارباً) أو لربط الذباب الاصطناعي (تسمح خرزات التنگستن للذبابة بالغرق بسرعة). يُستخدم التنگستن أيضاً كمسمار ثقيل لخفض معدل إطلاق النار في مدفع رشاش SWD M11/9 من 1.300 طلقة في الدقيقة إلى 700 طلقة في الدقيقة. تتضمن بعض أوتار الآلات الوترية التنگستن.[95][96] يستخدم التنگستن كمادة ماصة في تلسكوب الإلكترون الموجود على نظام الأشعة الكونية في مركبتي ڤويدجر الفضائيتين.[97]
كبديل للذهب
تسمح كثافة التنگستن المشابهة لكثافة الذهب، باستخدامه في صناعة المجوهرات كبديل للذهب أو الپلاتين.[17][98] التنگستن الفلزي هو أقل حساسية، وأصلب من سبائك الذهب (وإن لم يكن بنفس صلابة كربيد التنگستن)، مما يجعله مفيداً في صنع الخواتم المقاومة للخدش، خاصة في التصاميم ذات اللمسة النهائية المصقولة.
نظراً لأن كثافة التنگستن مشابهة جداً لكثافة الذهب (فهو أقل كثافة بنسبة 0.36% فقط)، وسعره الذي يبلغ جزءاً من ألف من سعر الذهب، يمكن أيضاً استخدام التنگستن في تقليد سبائك الذهب، مثل طلاء سبيكة التنگستن بالذهب،[99][100][101] الذي لوحظ منذ الثمانينيات،[102] أو أخذ سبيكة ذهبية موجودة، وحفر ثقوب فيها، واستبدال الذهب المستخرج بقضبان من التنگستن.[103] الكثافة ليست متطابقة تماماً، وتختلف خصائص الذهب والتنگستن الأخرى، لكن التنگستن المطلي بالذهب سيجتاز الاختبارات السطحية.[99]
يتوفر التنگستن المطلي بالذهب تجارياً من الصين (المصدر الرئيسي للتنگستن)، سواء في المجوهرات أو على شكل سبائك.[104]
الإلكترونيات
نظراً لاحتفاظه بمقاومته في درجات الحرارة العالية وتمتعه نقطة انصهار عالية، يُستخدم عنصر التنگستن في العديد من التطبيقات ذات درجات الحرارة العالية،[105] مثل خيوط المصابيح المتوهجة، وصمامات الأشعة المهبطية، والصمامات المفرغة، وعناصر التسخين، وفوهات المحركات الصاروخية.[17] كما أن درجة انصهاره العالية تجعل التنگستن مناسباً للاستخدامات الفضائية والاستخدامات ذات درجات الحرارة العالية مثل التطبيقات الكهربائية والتدفئة واللحام، لا سيما في عملية اللحام القوسية بالتنگستن الغازي (المعروفة أيضاً باسم لحام التنجستن بالغاز الخامل (TIG)).[106]
بسبب خصائصه الموصلة وخموله الكيميائي النسبي، يُستخدم التنگستن أيضاً في الأقطاب الكهربائية، وفي رؤوس الباعثات في أجهزة حزم الإلكترونات التي تستخدم مدافع انبعاث الحقل، مثل المجاهر الإلكترونية. في الإلكترونيات، يُستخدم التنگستن كمادة توصيل في الدوائر المتكاملة، بين مادة ثاني أكسيد السليكون العازلة كهربائياً والترانزستورات. كما يُستخدم في الأغشية المعدنية، التي تحل محل الأسلاك المستخدمة في الإلكترونيات التقليدية بطبقة من التنگستن (أو المولبدنم) على السليكون.[71]
إن التركيب الإلكتروني للتنگستن يجعله أحد المصادر الرئيسية لأهداف الأشعة السينية،[107][108] كما يُستخدم التنگستن للحماية من الإشعاعات عالية الطاقة (كما هو الحال في صناعة المستحضرات الصيدلانية الإشعاعية لحماية العينات المشعة من FDG). كما يُستخدم في التصوير بآشعة گاما كمادة لصنع فتحات مشفرة، نظراً لخصائصه الممتازة في الحماية. يُستخدم مسحوق التنگستن كمادة مالئة في مركبات اللدائن، التي تُستخدم بدورها كبديل غير سام للرصاص في الطلقات، المقذوفات، والدروع الواقية من الإشعاع. ولأن تمدده الحراري مشابه لتمدد زجاج البوروسيليكات، فإنه يُستخدم في صناعة وصلات الزجاج-الفلز.[20] إضافةً إلى ارتفاع درجة انصهاره، يؤدي تطعيم التنگستن بالپوتاسيوم إلى زيادة استقرار شكله (مقارنةً بالتنگستن غير المُطعّم). وهذا يضمن عدم ترهل الخيط، وعدم حدوث أي تغييرات غير مرغوب فيها.[109]
يُستخدم التنگستن في إنتاج محركات الاهتزاز، والمعروفة أيضاً باسم الهزازات المتنقلة.[110] تُعد هذه المحركات مكونات أساسية توفر ردود فعل لمسية للمستخدمين، لتنبيههم بالمكالمات والرسائل والإشعارات الواردة.[111] تساعد الكثافة العالية والتنگستن وصلابته ومقاومته للتآكل على تحمل الاهتزازات الدورانية عالية السرعة التي تولدها هذه المحركات.[112][113]
الأسلاك النانوية
من خلال عمليات التصنيع النانوي من أعلى إلى أسفل، تم تصنيع ودراسة أسلاك التنگستن النانوية منذ عام 2002.[114] بسبب النسبة العالية خاصة بين مساحة السطح والحجم، وتكوين طبقة أكسيد سطحية، وطبيعة البلورة الأحادية لهذه المادة، تختلف الخصائص الميكانيكية اختلافاً جوهرياً عن خصائص التنگستن الكتلي.[115] تتمتع هذه الأسلاك النانوية المصنوعة من التنگستن بتطبيقات محتملة في مجال الإلكترونيات النانوية، والأهم من ذلك، كمجسات لقياس درجة الحموضة ومجسات للغازات.[116] على غرار أسلاك السليكون النانوية، تُنتج أسلاك التنگستن النانوية غالباً من مادة أولية من التنگستن تليها خطوة أكسدة حرارية للتحكم في شكلها من حيث الطول ونسبة العرض إلى الطول.[117] باستخدام نموذج ديل-گروڤ من الممكن التنبؤ بحركية الأكسدة للأسلاك النانوية المصنعة من خلال عملية الأكسدة الحرارية هذه.[118]
طاقة الاندماج
نظراً لارتفاع درجة انصهاره ومقاومته الجيدة للتآكل، يُعدّ التنگستن خياراً رئيسياً للأجزاء الأكثر عرضة للپلازما في الجدار الداخلي لمفاعلات الاندماج النووي. يتميز التنگستن، كمادة مُكوّنة مُلامسة للپلازما، بانخفاض استثنائي في احتباس التريتيوم من خلال الترسيب المشترك والغرس، مما يُعزز السلامة بتقليل المخزون الإشعاعي، ويُحسّن كفاءة الوقود بتوفير المزيد منه لتفاعلات الاندماج، ويدعم استمرارية التشغيل بتقليل الحاجة إلى إزالة الوقود بشكل متكرر من الأسطح.[119] سيستخدم التنگستن كمادة مواجهة للپلازما في المحول في المفاعل النووي الحراري التجريبي الدولي (ITER)،[120] ويستخدم حالياً في مفاعل توروس الأوروپي المشترك التجريبي (JET).
الدور الحيوي
التنگستن، ذو العدد الذري Z = 74، هو أثقل عنصر معروف بدوره الحيوي. تستخدمه بعض أنواع الجراثيم والعتائق،[121] لكن ليس في حقيقيات النوى. على سبيل المثال، تستخدم إنزيمات تُسمى أوكسيدوريدوكتاز التنگستن بطريقة مشابهة للمولبدنم، وذلك باستخدامه في مُركب التنگستن-پترين مع الموليبدوپترين (على الرغم من اسمه، إلا أنه لا يحتوي على المولبدنم، ولكنه قد يُركب مع المولبدنم أو التنگستن عند استخدامه من قِبل العضيات). عادةً ما تُختزل الإنزيمات التي تستخدم التنگستن الأحماض الكربوكسيلية إلى ألدهيدات.[122] قد تحفز إنزيمات الأكسدة والاختزال بالتنگستن أيضاً عمليات الأكسدة. ويتطلب أول إنزيم تم اكتشافه ويحتاج إلى التنگستن السلينيوم أيضاً، وفي هذه الحالة، قد يعمل زوج التنگستن-سلينيوم بشكل مشابه لزوج المولبدنم-كبريت في بعض الإنزيمات التي تتطلب الموليبدوپترين.[123] من المعروف أن أحد الإنزيمات في عائلة الأكسيدوريدوكتاز التي تستخدم التنگستن أحياناً (إنزيم نازع هيدروجين الفورمات الجرثومي H) يستخدم نسخة من السلينيوم والمولبدنم من الموليبدوپترين.[124] إنزيم هيدراتاز الأسيتيلين هو إنزيم فلزي غير عادي لأنه يحفز تفاعل الإماهة. وقد اقتُرحت آليتان للتفاعل، إحداهما تتضمن تفاعلاً مباشراً بين ذرة التنگستن والرابطة الثلاثية C≡C.[125] على الرغم من العثور على إنزيم زانثين ديهيدروگنيز المحتوي على التنگستن من الجراثيم يحتوي على تنگستن-موليبدوپترين وأيضاً سلينيوم غير مرتبط بالپروتين، إلا أنه لم يُوصف مركب موليبدوپترين التنگستن-سلينيوم بشكل نهائي.[126]
في التربة، يتأكسد معدن التنگستن إلى أيون التنجستات. ويمكن لبعض العضيات بدائية النواة استيراده بشكل انتقائي أو غير انتقائي، وقد يحل محل المولبدات في بعض الإنزيمات. ويكون تأثيره على عمل هذه الإنزيمات مثبطاً في بعض الحالات، ومحفزاً في حالات أخرى.[127] تحدد التركيبة الكيميائية للتربة كيفية پلمرة التنگستن؛ فالتربة القلوية تُنتج تنگستات أحادية؛ والتربة الحمضية تُنتج تنگستات پوليمرية.[128]
تمت دراسة تأثير كل من تنگستات الصوديوم والرصاص على ديدان الأرض. ووجد أن الرصاص قاتل بتركيزات منخفضة، بينما كان تنگستات الصوديوم أقل سمية بكثير، إلا أن التنگستات تثبط قدرتها على التكاثر تماماً.[129]
خضع التنگستن للدراسة كضادات لأيض النحاس، في دور مشابه لعمل المولبدات. وقد وُجد أن أملاح رباعي ثيوتنگستات يمكن استخدامها كمواد كيميائية حيوية لتمخلب النحاس، على غرار رباعي ثيوموليبدات.[130]
الارتباط الوبائي المبكر بالسرطان
في 20 أغسطس 2002، أعلن مسؤولون يمثلون مراكز السيطرة على الأمراض والوقاية منها في الولايات المتحدة أن فحوصات البول التي أجريت على عائلات مرضى سرطان الدم وعائلات المجموعة الضابطة في فالون، نـِڤادا، أظهرت ارتفاع مستويات التنگستن في أجسام المجموعتين.[131] أُكتشفت ست عشرة حالة إصابة حديثة بالسرطان لدى الأطفال في فالون، والتي صُنفت الآن على أنها بؤرة لتفشي السرطان؛ على الرغم من أن غالبية المصابين بالسرطان ليسوا من سكان فالون القدامى. ومع ذلك، لا توجد بيانات كافية في الوقت الحالي لدعم وجود صلة بين التنگستن وسرطان الدم.[132]
في العتائق
يُعدّ التنگستن عنصراً أساسياً لبعض أنواع العتائق. ومن المعروف وجود الإنزيمات التالية التي تستخدم التنگستن:
- أوكسيدوريدوكتاز ألدهيد فريدوكسين (AOR) في Thermococcus strain ES-1
- الفورمالدهيد ferredoxin oxidoreductase (FOR) في Thermococcus litoralis
- Glyceraldehyde-3-phosphate ferredoxin oxidoreductase (GAPOR) في Pyrococcus furiosus
wtp هو نظام يُعرف نقل التنگستن بشكل انتقائي في العتائق:
- WtpA هو پروتين مرتبط بالتنگستن ضمن عائلة ناقلات ABC
- WtpB هو پرميز
- WtpC هو إنزيم الأدينوسين ثلاثي الفوسفات[133]
العوامل الصحية
لأن التنگستن فلز نادر،[134] ونظراً لأن مركباته خاملة بشكل عام، فإن تأثيرات التنگستن على البيئة محدودة.[135] يُعتقد أن وفرة التنگستن في القشرة الأرضية تبلغ حوالي 1.5 جزء في المليون. وهو العنصر رقم 58 من حيث الوفرة الموجودة على سطح الأرض.[136]
كان يُعتقد في البداية أنه فلز خامل نسبياً وسام بشكل طفيف فقط، لكن بدءاً من عام 2000، تم تسليط الضوء على المخاطر التي تشكلها سبائك التنگستن وغبارها وجزيئاتها في التسبب بالسرطان والعديد من الآثار الضارة الأخرى في الحيوانات وكذلك البشر من خلال التجارب المعملية والحيوية.[137][138] تعتمد الجرعة المميتة للنصيف LD50 بشكل كبير على الحيوان وطريقة الإعطاء وتتراوح بين 59 ملجم/كجم (عن طريق الوريد، للأرانب).[139][140] و5000 ملجم/كجم (مسحوق التنگستن الفلزي، داخل الصفاق، الجرذان).[141][142]
قد يتعرض الأفراد للتنگستن في أماكن العمل عن طريق استنشاقه، أو ابتلاعه، أو ملامسته للجلد، أو ملامسته للعينين. وقد حدد المعهد الوطني للسلامة والصحة المهنية حد التعرض الموصى به قدره 5 ملجم/م³ خلال يوم عمل مدته 8 ساعات، وحداً قصير الأجل قدره 10 ملجم/م³.[143]
احتياطات السلامة
يقاوم التنگستن معدن المولبدنم استنشاق هذا المعدن يسبب استثارة للرئة والغشاء المخاطى. استثارة العين يسفر عنها احمرار ودموع.التهاب الجلد: الاحمرار والهرش ووجود الندبات.التعرض المتكرر أو على المدى الطويل: لا يوجد هناك ما يفيد تدهور أياً من الحالات الطبية المرضية. لا توجد هناك أية نتائج تشير إلى حدوث تسمم التنجستين من الأطعمة.[144]
مزاعم ببراءة الإختراع
انظر أيضاً
- مدفع انبعاث الحقل
- حقل هاتشز كريك ولفرام، في الإقليم الشمالي (أستراليا)
- أكسيد التنگستن
- قائمة أصول أسماء العناصر الكيميائية
- قائمة الجدالات المتعلقة بتسمية العناصر الكيميائية
- العم تنجستن بقلم أوليڤر ساكس
المصادر
- ^ Berger, Dan. "Why does Tungsten not 'Kick' up an electron from the s sublevel ?". Bluffton College, USA.
- ^ Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. p. 6-134. ISBN 978-1-4200-9084-0.
- ^ Tolias P. (2017). "Analytical expressions for thermophysical properties of solid and liquid tungsten relevant for fusion applications". Nuclear Materials and Energy. 13: 42–57. arXiv:1703.06302. Bibcode:2017arXiv170306302T. doi:10.1016/j.nme.2017.08.002. S2CID 99610871.
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds" (PDF). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 978-0-8493-0486-6. Archived from the original (PDF) on 2011-03-03.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. p. E110. ISBN 978-0-8493-0464-4.
- ^ "Tungsten". Royal Society of Chemistry. Royal Society of Chemistry. Retrieved May 2, 2020.
- ^ أ ب van der Krogt, Peter. "Wolframium Wolfram Tungsten". Elementymology& Elements Multidict. Archived from the original on 2010-01-23. Retrieved 2010-03-11.
- ^ Münster, A.; Sivers, M. v.; Angloher, G.; Bento, A.; Bucci, C.; Canonica, L.; Erb, A.; Feilitzsch, F. v.; Gorla, P.; Gütlein, A.; Hauff, D.; Jochum, J.; Kraus, H.; Lanfranchi, J. -C.; Laubenstein, M.; Loebell, J.; Ortigoza, Y.; Petricca, F.; Potzel, W.; Pröbst, F.; Puimedon, J.; Reindl, F.; Roth, S.; Rottler, K.; Sailer, C.; Schäffner, K.; Schieck, J.; Scholl, S.; Schönert, S.; Seidel, W.; Stodolsky, L.; Strandhagen, C.; Strauss, R.; Tanzke, A.; Uffinger, M.; Ulrich, A.; Usherov, I.; Wawoczny, S.; Willers, M.; Wüstrich, M.; Zöller, A. (May 2014). "Radiopurity of CaWO4 crystals for direct dark matter search with CRESST and EURECA". Journal of Cosmology and Astroparticle Physics (05). arXiv:1403.5114. Bibcode:2014JCAP...05..018M. doi:10.1088/1475-7516/2014/05/018. 018.
- ^ أ ب ت Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ "wolfram" on Merriam-Webster.
- ^ "wolfram" on Oxford Dictionaries.
- ^ Zhang Y; Evans JRG and Zhang S (January 2011). "Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks". J. Chem. Eng. Data. 56 (2): 328–337. doi:10.1021/je1011086.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "This Chinese Company Proudly Sells Tungsten-Filled Gold Bars". Business Insider. 2012.
- ^ أ ب ت ث Daintith, John (2005). Facts on File Dictionary of Chemistry (4th ed.). New York: Checkmark Books. ISBN 978-0-8160-5649-1.
- ^ Lassner, Erik; Schubert, Wolf-Dieter (1999). "low temperature brittleness". Tungsten: properties, chemistry, technology of the element, alloys, and chemical compounds. Springer. pp. 20–21. ISBN 978-0-306-45053-2.
- ^ Gludovatz, B.; Wurster, S.; Weingärtner, T.; Hoffmann, A.; Pippan, R. (2011). "Influence of impurities on the fracture behavior of tungsten". Philosophical Magazine (Submitted manuscript). 91 (22): 3006–3020. Bibcode:2011PMag...91.3006G. doi:10.1080/14786435.2011.558861. S2CID 137145004.
- ^ أ ب ت ث ج Stwertka, Albert (2002). A Guide to the elements (2nd ed.). New York: Oxford University Press. ISBN 978-0-19-515026-1.
- ^ McMaster, J. & Enemark, John H. (1998). "The active sites of molybdenum- and tungsten-containing enzymes". Current Opinion in Chemical Biology. 2 (2): 201–207. doi:10.1016/S1367-5931(98)80061-6. PMID 9667924.
- ^ Hille, Russ (2002). "Molybdenum and tungsten in biology". Trends in Biochemical Sciences. 27 (7): 360–367. doi:10.1016/S0968-0004(02)02107-2. PMID 12114025.
- ^ أ ب ت Hammond, C. R. (2004). The Elements, in Handbook of Chemistry and Physics (81st ed.). CRC press. ISBN 978-0-8493-0485-9.
- ^ McMahon, Malcolm I.; Nelmes, Richard J. (2006). "High-pressure structures and phase transformations in elemental metals". Chemical Society Reviews (in الإنجليزية). 35 (10): 943–963. Bibcode:2006CSRev..35..943M. doi:10.1039/b517777b. ISSN 0306-0012. PMID 17003900.
- ^ Lassner, Erik; Schubert, Wolf-Dieter (1999). Tungsten: properties, chemistry, technology of the element, alloys, and chemical compounds. Springer. p. 9. ISBN 978-0-306-45053-2.
- ^ Bean, Heather (October 19, 1998). Material Properties and Analysis Techniques for Tungsten Thin Films. frii.com
- ^ Lita, A. E.; Rosenberg, D.; Nam, S.; Miller, A.; Balzar, D.; Kaatz, L. M.; Schwall, R. E. (2005). "Tuning of Tungsten Thin Film Superconducting Transition Temperature for Fabrication of Photon Number Resolving Detectors" (PDF). IEEE Transactions on Applied Superconductivity. 15 (2): 3528–3531. Bibcode:2005ITAS...15.3528L. doi:10.1109/TASC.2005.849033. S2CID 5804011. Archived (PDF) from the original on 2013-05-13.
- ^ Johnson, R. T.; O. E. Vilches; J. C. Wheatley; Suso Gygax (1966). "Superconductivity of Tungsten". Physical Review Letters. 16 (3): 101–104. Bibcode:1966PhRvL..16..101J. doi:10.1103/PhysRevLett.16.101.
- ^ Autler, S. H.; J. K. Hulm; R. S. Kemper (1965). "Superconducting Technetium–Tungsten Alloys". Physical Review. 140 (4A): A1177–A1180. Bibcode:1965PhRv..140.1177A. doi:10.1103/PhysRev.140.A1177.
- ^ Shailos, A.; W Nativel; A Kasumov; C Collet; M Ferrier; S Guéron; R Deblock; H Bouchiat (2007). "Proximity effect and multiple Andreev reflections in few-layer graphene". Europhysics Letters. 79 (5) 57008. arXiv:cond-mat/0612058. Bibcode:2007EL.....7957008S. doi:10.1209/0295-5075/79/57008. S2CID 119351442.
- ^ Kasumov, A. Yu.; K. Tsukagoshi; M. Kawamura; T. Kobayashi; Y. Aoyagi; K. Senba; T. Kodama; H. Nishikawa; I. Ikemoto; K. Kikuchi; V. T. Volkov; Yu. A. Kasumov; R. Deblock; S. Guéron; H. Bouchiat (2005). "Proximity effect in a superconductor-metallofullerene-superconductor molecular junction". Physical Review B. 72 (3) 033414. arXiv:cond-mat/0402312. Bibcode:2005PhRvB..72c3414K. doi:10.1103/PhysRevB.72.033414. S2CID 54624704.
- ^ Kirk, M. D.; D. P. E. Smith; D. B. Mitzi; J. Z. Sun; D. J. Webb; K. Char; M. R. Hahn; M. Naito; B. Oh; M. R. Beasley; T. H. Geballe; R. H. Hammond; A. Kapitulnik; C. F. Quate (1987). "Point-contact electron tunneling into the high-T_{c} superconductor Y-Ba-Cu-O". Physical Review B. 35 (16): 8850–8852. Bibcode:1987PhRvB..35.8850K. doi:10.1103/PhysRevB.35.8850. PMID 9941272.
- ^ Danevich, F. A.; et al. (2003). "α activity of natural tungsten isotopes". Phys. Rev. C. 67 (1) 014310. arXiv:nucl-ex/0211013. Bibcode:2003PhRvC..67a4310D. doi:10.1103/PhysRevC.67.014310. S2CID 6733875.
- ^ Cozzini, C.; et al. (2004). "Detection of the natural α decay of tungsten". Phys. Rev. C. 70 (6) 064606. arXiv:nucl-ex/0408006. Bibcode:2004PhRvC..70f4606C. doi:10.1103/PhysRevC.70.064606. S2CID 118891861.
- ^ أ ب ت ث ج ح Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brookhaven National Laboratory. Archived from the original on 2008-05-22. Retrieved 2008-06-06.
- ^ "Tungsten: reactions of elements".
- ^ أ ب Emsley, John E. (1991). The elements (2nd ed.). New York: Oxford University Press. ISBN 978-0-19-855569-8.
- ^ Morse, P. M.; Shelby, Q. D.; Kim, D. Y.; Girolami, G. S. (2008). "Ethylene Complexes of the Early Transition Metals: Crystal Structures of [HfEt4(C2H4)2−] and the Negative-Oxidation-State Species [TaHEt(C2H4)33−] and [WH(C2H4)43−]". Organometallics. 27 (5): 984–993. doi:10.1021/om701189e.
- ^ Smith, Bradley J.; Patrick, Vincent A. (2000). "Quantitative Determination of Sodium Metatungstate Speciation by 183W N.M.R. Spectroscopy". Australian Journal of Chemistry. 53 (12): 965. doi:10.1071/CH00140.
- ^ Borin, Antonio Carlos; Gobbo, João Paulo; Roos, Björn O. (January 2008). "A theoretical study of the binding and electronic spectrum of the Mo2 molecule". Chemical Physics. 343 (2–3): 210–216. Bibcode:2008CP....343..210B. doi:10.1016/j.chemphys.2007.05.028. ISSN 0301-0104.
- ^ Roos, Björn O.; Borin, Antonio C.; Laura Gagliardi (2007). "Reaching the Maximum Multiplicity of the Covalent Chemical Bond". Angew. Chem. Int. Ed. 46 (9): 1469–72. Bibcode:2007ACIE...46.1469R. doi:10.1002/anie.200603600. PMID 17225237.
- ^ Scheele, Carl Wilhelm (1781) "Tungstens bestånds-delar" (Tungsten's constituents), Kungliga Vetenskaps Academiens Nya Handlingar (Royal Scientific Academy's New Proceedings), 2 : 89–95 (in Swedish).
- ^ English translation on pp. 4–13 of: de Luyart, John Joseph and Fausto, with Charles Cullen, trans., A Chemical Analysis of Wolfram and Examination of a New Metal, Which Enters its Composition (London, England, G. Nicol, 1785).
- ^ أ ب Saunders, Nigel (2004). Tungsten and the Elements of Groups 3 to 7 (The Periodic Table). Chicago, Illinois: Heinemann Library. ISBN 978-1-4034-3518-7.
- ^ "ITIA Newsletter" (PDF). International Tungsten Industry Association. June 2005. Archived from the original on July 21, 2011. Retrieved 2008-06-18.
{{cite news}}: CS1 maint: unfit URL (link) - ^ "ITIA Newsletter" (PDF). International Tungsten Industry Association. December 2005. Archived from the original on July 21, 2011. Retrieved 2008-06-18.
{{cite news}}: CS1 maint: unfit URL (link) - ^ de Luyart, J.J. and F. (September 1783) "Análisis químico del volfram, y examen de un nuevo metal, que entra en su composición" (Chemical analysis of wolframite, and examination of a new metal, which enters into its composition), Extractos de las Juntas Generales celebradas por la Real Sociedad Bascongada de los Amigos del País en la ciudad de Vitoria por setiembre de 1783, pp. 46–88.
- ^ de Luyart, John Joseph and Fausto, with Charles Cullen, trans., A Chemical Analysis of Wolfram and Examination of a New Metal, Which Enters its Composition (London, England, G. Nicol, 1785).
- ^ Caswell, Lyman R. and Stone Daley, Rebecca W. (1999) "The Delhuyar brothers, tungsten, and Spanish silver," Bulletin for the History of Chemistry, 23 : 11–19. Available at: University of Illinois (USA) Archived 2015-12-30 at the Wayback Machine
- ^ Watson, Greig (2014-06-06). "Vital WW1 metal 'in enemy hands'". BBC News. Retrieved 2018-02-10.
- ^ Stevens, Donald G. (1999). "World War II Economic Warfare: The United States, Britain, and Portuguese Wolfram". The Historian. 61 (3): 539. doi:10.1111/j.1540-6563.1999.tb01036.x.
- ^ Wheeler, L. Douglas (Summer 1986). "The Price of Neutrality: Portugal, the Wolfram Question, and World War II". Luso-Brazilian Review. 23 (1): 107–127. JSTOR 3513391.
- ^ General Electric Co. v. De Forest Radio Co., 28 F.2d 641, 643 (3rd Cir. 1928)
- ^ Guruswamy, Lakshman D.; McNeely, Jeffrey A. (1998). Protection of global biodiversity: converging strategies. Duke University Press. pp. 333–. ISBN 978-0-8223-2188-0.
- ^ General Electric Co. v. De Forest Radio Co., 28 F.2d 641 (3d Cir. خطأ: لا توجد وحدة بهذا الاسم "auto date formatter".).
- ^ "Chemical analyses find hidden elements from renaissance astronomer Tycho Brahe's alchemy laboratory". Phys.org. 24 July 2024. Retrieved 2 March 2025.
- ^ أ ب "Tungsten, W, atomic number 74". Institute of rare earths elements and strategic metals.
- ^ Danevich, F. A.; et al. (2003). "α activity of natural tungsten isotopes". Phys. Rev. C. 67 (1) 014310. arXiv:nucl-ex/0211013. Bibcode:2003PhRvC..67a4310D. doi:10.1103/PhysRevC.67.014310. S2CID 6733875.
- ^ Cozzini, C.; et al. (2004). "Detection of the natural α decay of tungsten". Phys. Rev. C. 70 (6) 064606. arXiv:nucl-ex/0408006. Bibcode:2004PhRvC..70f4606C. doi:10.1103/PhysRevC.70.064606. S2CID 118891861.
- ^ راتب محملجي. "التنغستِن". الموسوعة العربية.
- ^ "supply-circularity". itia.info.
- ^ Tungsten. Mineral Commodity Summaries. USGS (2017)
- ^ أ ب Shedd, Kim B. (December 2018) Tungsten. 2016 Minerals Yearbook. USGS
- ^ Tungsten. Mineral Commodity Summaries. USGS (2018)
- ^ "Work starts on £130m Devon tungsten mine". BBC News. 9 June 2014. Archived from the original on 2014-12-05.
- ^ "How Hemerdon mine lost £100m in just three years". Plymouth Herald. 12 October 2018. Retrieved 24 January 2019.
- ^ Altenberger, Florian; Raith, Johann G.; Weilbold, Julia; Auer, Christian; Knoll, Tanja; Paulick, Holger; Schedl, Albert; Aupers, Karsten; Schmidt, Steffen; Neinavaie, Hassan (2021-05-07). "Casting new light on tungsten deposits in the Eastern Alps". Zeitschrift der Deutschen Gesellschaft für Geowissenschaften (in الإنجليزية). 172 (1): 63–72. Bibcode:2021ZDGG..172...63A. doi:10.1127/zdgg/2021/0262. S2CID 233912162.
- ^ Mateus, António; Lopes, Catarina; Martins, Luís; Gonçalves, Mário Abel (June 2021). "Current and Foreseen Tungsten Production in Portugal, and the Need of Safeguarding the Access to Relevant Known Resources". Resources (in الإنجليزية). 10 (6): 64. Bibcode:2021Resou..10...64M. doi:10.3390/resources10060064. hdl:10451/53675. ISSN 2079-9276.
- ^ Kristof, Nicholas D. (2010-06-27). "Death by Gadget". The New York Times. Archived from the original on 2016-08-31.
- ^ "The Genocide Behind Your Smart Phone". The Daily Beast. July 16, 2010. Archived from the original on 2011-11-17.
- ^ "Project Technical Reports – Sangdong Reserves/Resources NI 43-101" (PDF). Almonty Industries. 31 July 2016. Archived from the original (PDF) on 12 September 2024. Retrieved 12 September 2024.
- ^ "Almonty advances Sangdong tungsten mine in South Korea". The Northern Miner. 2 August 2023. Archived from the original on 28 April 2024. Retrieved 28 April 2024.
- ^ Shanaka Anslem Perera (2025-12-27). "I. THE FORM THAT NEVER COMES BACK". substack.com.
- ^ أ ب Schey, John A. (1987). Introduction to Manufacturing Processes (2nd ed.). McGraw-Hill, Inc.
- ^ "Tungsten Pricing". International Tungsten Industry Association. Retrieved 18 June 2020.
- ^ Trasorras, Juan R. L.; Wolfe, Thomas A.; Knabl, Wolfram; Venezia, Carmen; Lemus, Ranulfo; Lassner, Erik; Schubert, Wolf-Dieter; Lüderitz, Eberhard; Wolf, Hans-Uwe (2016). "Tungsten, Tungsten Alloys, and Tungsten Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a27_229.pub2. ISBN 9783527303854.
- ^ Tan, C. (2018). "Selective laser melting of high-performance pure tungsten: parameter design, densification behavior and mechanical properties". Sci. Technol. Adv. Mater. 19 (1): 370–380. Bibcode:2018STAdM..19..370T. doi:10.1080/14686996.2018.1455154. PMC 5917440. PMID 29707073.
- ^ Don Law-West; Louis Perron. "Tungsten". The Canadian Encyclopaedia. Retrieved 2020-07-18.
- ^ Tungsten: The Element, History, Uses and Wedding Bands.tungstenworld.com
- ^ de Laubenfels, Blair; Weber, Christy; Bamberg, Kim (2009). Knack Planning Your Wedding: A Step-by-Step Guide to Creating Your Perfect Day. Globe Pequot. pp. 35–. ISBN 978-1-59921-397-2.
- ^ Schultz, Ken (2009). Ken Schultz's Essentials of Fishing: The Only Guide You Need to Catch Freshwater and Saltwater Fish. John Wiley and Sons. pp. 138–. ISBN 978-0-470-44431-3.
- ^ "Tungsten Applications – Steel". Azom. 2000–2008. Archived from the original on 2008-08-15. Retrieved 2008-06-18.
- ^ Ramakrishnan, P. (2007). "Powder metallurgy for Aerospace Applications". Powder Metallurgy: Processing for automotive, electrical / electronic, and engineering industry. New Age International. p. 38. ISBN 978-81-224-2030-2.
- ^ "Tungsten Applications". wolfmet.com. Archived from the original on 2013-09-01.
- ^ "TIG Torches & TIG Torch Parts". AES Industrial Supplies Limited (in الإنجليزية البريطانية). Retrieved 2021-05-06.
- ^ Dense Inert Metal Explosive (DIME). Defense-update.com. Retrieved on 2011-08-07.
- ^ Delmon, Bernard & Froment, Gilbert F. (1999). Hydrotreatment and hydrocracking of oil fractions: proceedings of the 2nd international symposium, 7th European workshop, Antwerpen, Belgium, November 14–17, 1999. Elsevier. pp. 351–. ISBN 978-0-444-50214-8. Retrieved 18 December 2011.
- ^ Mang, Theo & Dresel, Wilfried (2007). Lubricants and Lubrication. John Wiley & Sons. pp. 695–. ISBN 978-3-527-61033-4.
- ^ Spivey, James J. (2002). Catalysis. Royal Society of Chemistry. pp. 239–. ISBN 978-0-85404-224-1. Retrieved 18 December 2011.
- ^ Lewandowski, Grzegorz; Kujbida, Marcin; Wróblewska, Agnieszka (1 April 2021). "Epoxidation of 1,5,9-cyclododecatriene with hydrogen peroxide under phase-transfer catalysis conditions: influence of selected parameters on the course of epoxidation". Reaction Kinetics, Mechanisms and Catalysis (in الإنجليزية). 132 (2): 983–1001. doi:10.1007/s11144-021-01960-7. ISSN 1878-5204.
- ^ Kinetic studies of propane oxidation on Mo and V based mixed oxide catalysts. 2011. pp. 165–170.
- ^ Liu, Lujie; Asano, Takehiro; Nakagawa, Yoshinao; Gu, Minyan; Li, Congcong; Tamura, Masazumi; Tomishige, Keiichi (5 September 2021). "Structure and performance relationship of silica-supported platinum–tungsten catalysts in selective C-O hydrogenolysis of glycerol and 1,4-anhydroerythritol". Applied Catalysis B: Environmental. 292 120164. Bibcode:2021AppCB.29220164L. doi:10.1016/j.apcatb.2021.120164.
- ^ Kornas, A.; Śliwa, M.; Ruggiero-Mikołajczyk, M.; Samson, K.; Podobiński, J.; Karcz, R.; Duraczyńska, D.; Rutkowska-Zbik, D.; Grabowski, R. (1 June 2020). "Direct hydrogenation of CO2 to dimethyl ether (DME) over hybrid catalysts containing CuO/ZrO2 as a metallic function and heteropolyacids as an acidic function". Reaction Kinetics, Mechanisms and Catalysis (in الإنجليزية). 130 (1): 179–194. doi:10.1007/s11144-020-01778-9. ISSN 1878-5204.
- ^ Campos, Willison E. O.; Lopes, Anna S. C.; Monteiro, Waldinei R.; Filho, Geraldo N. R.; Nobre, Francisco X.; Luz, Patrícia T. S.; Nascimento, Luís A. S.; Costa, Carlos E. F.; Monteiro, Wesley F.; Vieira, Michele O.; Zamian, José R. (1 October 2020). "Layered double hydroxides as heterostructure LDH@Bi2WO6 oriented toward visible-light-driven applications: synthesis, characterization, and its photocatalytic properties". Reaction Kinetics, Mechanisms and Catalysis (in الإنجليزية). 131 (1): 505–524. doi:10.1007/s11144-020-01830-8. ISSN 1878-5204. S2CID 220948033.
- ^ Maslana, K.; Wenelska, K.; Biegun, M.; Mijowska, E. (5 June 2020). "High catalytic performance of tungsten disulphide rodes in oxygen evolution reactions in alkaline solutions". Applied Catalysis B: Environmental. 266 118575. Bibcode:2020AppCB.26618575M. doi:10.1016/j.apcatb.2019.118575. S2CID 213246090.
- ^ "F1 Technique: The secrets of ballast in a Formula 1 car". Auto123.com. 2013-12-25. Retrieved 2019-02-03.
- ^ Turrell, Kerry (2004). Tungsten. Marshall Cavendish. p. 24. ISBN 978-0-7614-1548-0.
- ^ Prieto, Carlos (2011-02-01). The Adventures of a Cello. Austin: University of Texas Press. p. 10. ISBN 978-0-292-72393-1.
- ^ Pickering N. C. (1991). The Bowed String: Observations on the Design, Manufacture, Testing and Performance of Strings for Violins, Violas and Cellos. Mattituck, New York: Amereon. pp. 5–6, 17.
- ^ "CRS Instruments". NASA. Archived from the original on 2017-02-01.
- ^ Hesse, Rayner W. (2007). "tungsten". Jewelrymaking through history: an encyclopedia. Westport, Conn.: Greenwood Press. pp. 190–192. ISBN 978-0-313-33507-5.
- ^ أ ب Gray, Theo (March 14, 2008). "How to Make Convincing Fake-Gold Bars". Popular Science. Archived from the original on December 29, 2014. Retrieved 2008-06-18.
- ^ "Zinc Dimes, Tungsten Gold & Lost Respect Archived 2011-10-08 at the Wayback Machine", Jim Willie, Nov 18 2009
- ^ "Largest Private Refinery Discovers Gold-Plated Tungsten Bar – Coin Update". news.coinupdate.com.
- ^ "Austrians Seize False Gold Tied to London Bullion Theft". The New York Times. Reuters. 1983-12-22. Archived from the original on 2012-03-27. Retrieved 2012-03-25.
- ^ Tungsten filled Gold bars Archived 2012-03-26 at the Wayback Machine, ABC Bullion, Thursday, March 22, 2012
- ^ Tungsten Alloy for Gold Substitution Archived 2012-03-22 at the Wayback Machine, China Tungsten
- ^ DeGarmo, E. Paul (1979). Materials and Processes in Manufacturing (5th ed.). New York: MacMillan Publishing.
- ^ Cary, Howard B.; Helzer, Scott (2005). Modern welding Technology (6th ed.). Upper Saddle River, NJ: Pearson/Prentice Hall. ISBN 978-0-13-113029-6.
- ^ Curry, Thomas S.; Dowdey, James E.; Murry, Robert C.; Christensen, Edward E. (1990-08-01). Christensen's physics of diagnostic radiology. Lippincott Williams & Wilkins. pp. 29–35. ISBN 978-0-8121-1310-5. Archived from the original on 2017-11-11.
- ^ Hasz, Wayne Charles et al. (August 6, 2002) "X-ray target" U.S. Patent 6٬428٬904
- ^ "Non-Sag Doped Tungsten – Union City Filament". Union City Filament. Archived from the original on 2017-05-01. Retrieved 2017-04-28.
- ^ Trento, Chin (Mar 8, 2024). "How Much Gold Can an iPhone Refine?". Stanford Advanced Materials. Retrieved June 26, 2024.
- ^ {{{1}}} patent {{{2}}}
- ^ Nissen, Nils F.; Reinhold, Julia (2021). "Recyclability of Tungsten, Tantalum and Neodymium from Smartphones". In Inoue, M.; Fukushige, S. (eds.). EcoDesign and Sustainability I: Products, Services, and Business Models. Springer Singapore. pp. 365–381. ISBN 978-981-15-6779-7.
- ^ Micallef, Christian; Zhuk, Yuri (2020). "Recent Progress in Precision Machining and Surface Finishing of Tungsten Carbide Hard Composite Coatings". Coatings. 10 (8): 731. doi:10.3390/coatings10080731.
- ^ Li Yadong (2002). "From Surfactant–Inorganic Mesostructures to Tungsten Nanowires". Angewandte Chemie. 114 (2): 333–335. Bibcode:2002AngCh.114..343L. doi:10.1002/1521-3773(20020118)41:2<333::AID-ANIE333>3.0.CO;2-5. PMID 12491423.
- ^ Volker Cimalla (2008). "Nanomechanics of single crystalline tungsten nanowires". Journal of Nanomaterials. 2008 638947: 1–9. doi:10.1155/2008/638947. hdl:11858/00-001M-0000-0019-4CC6-3.
- ^ CNR Rao (2006). "High-sensitivity hydrocarbon sensors based on tungsten oxide nanowires". Journal of Materials Chemistry.
- ^ Liu, M.; Peng, J.; et al. (2016). "Two-dimensional modeling of the self-limiting oxidation in silicon and tungsten nanowires". Theoretical and Applied Mechanics Letters. 6 (5): 195–199. arXiv:1911.08908. Bibcode:2016TAML....6..195L. doi:10.1016/j.taml.2016.08.002.
- ^ JTL Thong (2010). "Thermal oxidation of polycrystalline tungsten nanowire" (PDF). Journal of Applied Physics. 108 (9) 094312: 094312–094312–6. Bibcode:2010JAP...108i4312Y. doi:10.1063/1.3504248. Archived (PDF) from the original on 2017-03-15.
- ^ Roth, Joachim; Tsitrone, E.; Loarte, A.; Loarer, Th.; Counsell, G.; Neu, R.; Philipps, V.; Brezinsek, S.; Lehnen, M.; Coad, P.; Grisolia, Ch.; Schmid, K.; Krieger, K.; Kallenbach, A.; Lipschultz, B.; Doerner, R.; Causey, R.; Alimov, V.; Shu, W.; Ogorodnikova, O.; Kirschner, A.; Federici, G.; Kukushkin, A. (2009). "Recent analysis of key plasma wall interactions issues for ITER". Journal of Nuclear Materials. 390–391: 1–9. Bibcode:2009JNuM..390....1R. doi:10.1016/j.jnucmat.2009.01.037. hdl:11858/00-001M-0000-0026-F442-2. ISSN 0022-3115.
- ^ Pitts, R. A.; Carpentier, S.; Escourbiac, F.; Hirai, T.; Komarov, V.; Lisgo, S.; Kukushkin, A. S.; Loarte, A.; Merola, M.; Sashala Naik, A.; Mitteau, R. (2013-07-01). "A full tungsten divertor for ITER: Physics issues and design status". Journal of Nuclear Materials. Proceedings of the 20th International Conference on Plasma-Surface Interactions in Controlled Fusion Devices (in الإنجليزية). 438: S48–S56. Bibcode:2013JNuM..438S..48P. doi:10.1016/j.jnucmat.2013.01.008. ISSN 0022-3115.
- ^ Johnson JL, Rajagopalan KV, Mukund S, Adams MW (5 March 1993). "Identification of molybdopterin as the organic component of the tungsten cofactor in four enzymes from hyperthermophilic Archaea". Journal of Biological Chemistry. 268 (7): 4848–52. doi:10.1016/S0021-9258(18)53474-8. PMID 8444863.
- ^ Lassner, Erik (1999). Tungsten: Properties, Chemistry, Technology of the Element, Alloys and Chemical Compounds. Springer. pp. 409–411. ISBN 978-0-306-45053-2.
- ^ Stiefel, E. I. (1998). "Transition metal sulfur chemistry and its relevance to molybdenum and tungsten enzymes" (PDF). Pure Appl. Chem. 70 (4): 889–896. CiteSeerX 10.1.1.614.5712. doi:10.1351/pac199870040889. S2CID 98647064. Archived (PDF) from the original on 2008-12-03.
- ^ Khangulov, S. V.; et al. (1998). "Selenium-Containing Formate Dehydrogenase H from Escherichia coli: A Molybdopterin Enzyme That Catalyzes Formate Oxidation without Oxygen Transfer". Biochemistry. 37 (10): 3518–3528. doi:10.1021/bi972177k. PMID 9521673.
- ^ ten Brink, Felix (2014). "Chapter 2. Living on acetylene. A Primordial Energy Source". In Peter M.H. Kroneck; Martha E. Sosa Torres (eds.). The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences. Vol. 14. Springer. pp. 15–35. doi:10.1007/978-94-017-9269-1_2. ISBN 978-94-017-9268-4. PMID 25416389.
- ^ Schrader, Thomas; Rienhofer, Annette; Andreesen, Jan R. (1999). "Selenium-containing xanthine dehydrogenase from Eubacterium barkeri". Eur. J. Biochem. 264 (3): 862–71. doi:10.1046/j.1432-1327.1999.00678.x. PMID 10491134.
- ^ Andreesen, J. R.; Makdessi, K. (2008). "Tungsten, the Surprisingly Positively Acting Heavy Metal Element for Prokaryotes". Annals of the New York Academy of Sciences. 1125 (1): 215–229. Bibcode:2008NYASA1125..215A. doi:10.1196/annals.1419.003. PMID 18096847. S2CID 19459237.
- ^ Petkewich, Rachel A. (19 January 2009). "Unease over Tungsten". Chemical & Engineering News. 87 (3): 63–65. doi:10.1021/cen-v087n003.p063.
- ^ Inouye, L. S.; et al. (2006). "Tungsten effects on survival, growth, and reproduction in the earthworm, eisenia fetida". Environmental Toxicology and Chemistry. 25 (3): 763–8. Bibcode:2006EnvTC..25..763I. doi:10.1897/04-578R.1. PMID 16566161. S2CID 38620368.
- ^ McQuaid A; Lamand M; Mason J (1994). "Thiotungstate-copper interactions II. The effects of tetrathiotungstate on systemic copper metabolism in normal and copper-treated rats". J Inorg Biochem. 53 (3): 205–18. doi:10.1016/0162-0134(94)80005-7. PMID 8133256.
- ^ "Cross-Sectional Exposure Assessment of Environmental Contaminants in Churchill County, Nevada". Centers for Disease Control and Prevention. 2003-02-06. Retrieved 2008-05-09.
- ^ Mullen, Frank X. (April 27, 2006). "Mouse Study Findings key in Fallon Cancer Cases, Scientists Say". Reno Gazette-Journal. Retrieved 2008-06-17.
- ^ Paul Blum, ed. (1 April 2008). Archaea: New Models for Prokaryotic Biology. Caister Academic Press. ISBN 978-1-904455-27-1.
- ^ Brown, Mark (7 September 2011). "The Earth's most precious metals arrived on meteorites". wired.co.uk.
- ^ Strigul, N; Koutsospyros, A; Arienti, P; Christodoulatos, C; Dermatas, D; Braida, W (2005). "Effects of tungsten on environmental systems". Chemosphere. 61 (2): 248–58. Bibcode:2005Chmsp..61..248S. doi:10.1016/j.chemosphere.2005.01.083. PMID 16168748.
- ^ Krebs, Robert E. (2006-07-30). The History and Use of Our Earth's Chemical Elements: A Reference Guide (in الإنجليزية). Bloomsbury Publishing USA. ISBN 978-0-313-02798-7.
- ^ Laulicht, F.; Brocato, J.; Cartularo, L.; Vaughan, J.; Wu, F.; Vaughan, J.; Kluz, T.; Sun, H.; Oksuz, B. A.; Shen, S.; Peana, M.; Medici, S.; Zoroddu, M. A.; Costa, M. (2015). "Tungsten-induced carcinogenesis in human bronchial epithelial cells". Toxicology and Applied Pharmacology. 288 (1): 33–39. Bibcode:2015ToxAP.288...33L. doi:10.1016/j.taap.2015.07.003. PMC 4579035. PMID 26164860.
- ^ Zoroddu, M. A.; Medici, S.; Peana, M.; Nurchi, V. M.; Lachowicz, J. I.; Laulicht, J.; Costa, M. (2017). "Tungsten or Wolfram: Friend or Foe?". Curr. Med. Chem. 24 (1): 65–90. doi:10.2174/0929867324666170428105603. PMID 27855621.
- ^ Koutsospyros, A.; Braida, W.; Christodoulatos, C.; Dermatas, D.; Strigul, N. (2006). "A review of tungsten: From environmental obscurity to scrutiny". Journal of Hazardous Materials. 136 (1): 1–19. Bibcode:2006JHzM..136....1K. doi:10.1016/j.jhazmat.2005.11.007. PMID 16343746.
- ^ Lagarde, F.; Leroy, M. (2002). Metabolism and toxicity of tungsten in humans and animals. Metal Ions in Biological Systems. Vol. 39. pp. 741–59. doi:10.1201/9780203909331.ch22 (inactive 12 July 2025). ISBN 978-0-8247-0765-1. PMID 11913143.
{{cite book}}: CS1 maint: DOI inactive as of يوليو 2025 (link) also reported in Astrid Sigel; Helmut Sigel (2002). Molybdenum and tungsten: their roles in biological processes. CRC Press. p. 741 ff. ISBN 978-0-8247-0765-1. - ^ Masten, Scott (2003). "Tungsten and Selected Tungsten Compounds – Review of Toxicological Literature" (PDF). National Institute of Environmental Health Sciences. Archived from the original (PDF) on 2009-03-25. Retrieved 2009-03-19.
- ^ Marquet, P.; et al. (1997). "Tungsten determination in biological fluids, hair and nails by plasma emission spectrometry in a case of severe acute intoxication in man". Journal of Forensic Sciences. 42 (3): 527–30. doi:10.1520/JFS14162J. PMID 9144946.
- ^ "CDC – NIOSH Pocket Guide to Chemical Hazards – Tungsten". www.cdc.gov. Archived from the original on 2015-11-25. Retrieved 2015-11-24.
- ^ المعادن الثقيلة.. سموم بيئية، فيدو
وصلات خارجية
- CS1 maint: unfit URL
- CS1 الإنجليزية البريطانية-language sources (en-gb)
- CS1 maint: DOI inactive as of يوليو 2025
- Short description is different from Wikidata
- Pages using infobox element with unknown parameters
- Articles containing ألمانية-language text
- Articles containing لاتينية-language text
- Articles with hatnote templates targeting a nonexistent page
- Articles with unsourced statements from December 2022
- أملاح التنگستن
- صهر المعادن
- علم الأحياء وعلم الصيدلة من العناصر الكيميائية
- علوم وتكنولوجيا في اسبانيا
- عناصر كيميائية
- فلزات انتقالية
- مواد حرارية
- تنگستن







